Technologies
Preclinical Cardiovascular Imaging
Echocardiography
Measuring the cardiac function is indispensable in clinical and pre-clinical research.
The evaluation of the cardiac function using ultrasound (echocardiography) is performed in our small-animal ultrasound lab. Using an advanced ultra-high frequency preclinical ultrasound imaging system including color-, pulsed-wave- and tissue-doppler, we can measure all relevant functional parameters for the evaluation of e.g. systolic or diastolic dysfunction. Further analysis can be performed offline. Our group established reliable protocols for the ultrasound examination of experimental animals.
Equipment:
- Fujifilm VisualSonics Vevo 3000 Preclinical Imaging System
- state-of-the art animal anesthesia system
- corresponding offline-analysis suite
Publication: Revealing subtle changes in cardiac function using transthoracic dobutamine stress echocardiography in mice. Settelmeier S, Rassaf T, Hendgen-Cotta, UB. J Vis Exp. 2021;(168)
https://pubmed.ncbi.nlm.nih.gov/33645572/
Contact:
Dr. med.
Stephan Settelmeier

Invasive hemodynamics
In addition to non-invasive ultrasound measurements, we use invasive catheter-based real-time pressure-volume hemodynamic analysis for exact quantification of the cardiac function, which can be performed simultaneously to e.g. surgical procedures. Due to the interoperability, we are able to perform ultrasound-guided catheter-placement.
Equipment:
- Millar Research Pressure-Volume System
- Mikro-Tip Pressure Catheters in different sizes
Publication: Real-time pressure-volume analysis of acute myocardial infarction in mice. Michel L, Stock P, Rammos C, Totzeck M, Rassaf T, Hendgen-Cotta UB. J Vis Exp. 2018;(137):57621
https://www.jove.com/de/t/57621/real-time-pressure-volume-analysis-acute-myocardial-infarction
Contact:
PD Dr. med.
Lars Michel
Imaging
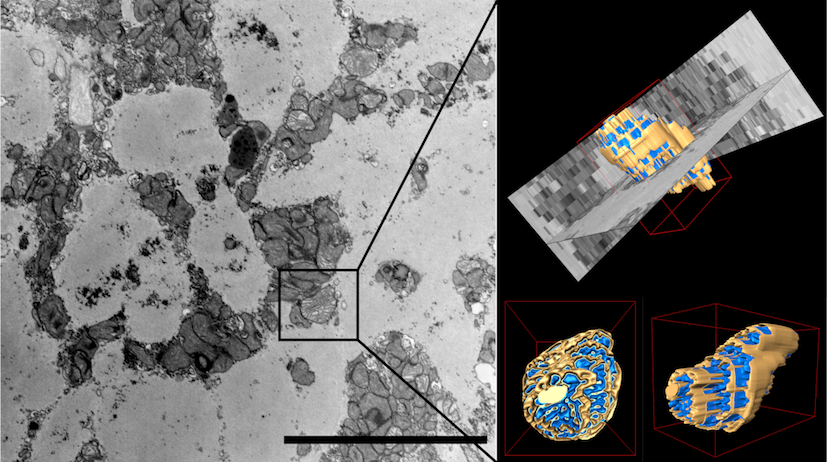
Electron microscopy
High-resolution electron microscopy is an important tool for illuminating the nanoworld and, in particular, enables the characterization of tissue, cells and organelles with distinct axial directions in indispensable 3D analysis. The 3D technique focused ion-beam scanning electron microscopy (FIB-SEM) combines the electron beam characteristic of SEM for „scanning“ the sample surface and a high-energy, focused (gallium) ion beam as a cutting tool. While the area to be analyzed is being continuously removed by the FIB, the electron beam generates surface images of the freshly cut sample. Image stacks often consist of several hundreds of single image planes, which can subsequently be reconstructed in silico into a 3D model offering a wide variety of parameter analyses.
Methods:
- Classic TEM and SEM analyses
- 3-dimensional nanotomography
- Tokuyasu technique for immunogold labeling
- Extension of the sample preparation by cryo-fixation
- Correlative Light and Electron Microscopy (CLEM)
- Light microscopy (widefield, confocal, superresolution) correlates with TEM or SEM
- Classic antibody staining
- Pre-embedding CLEM
The devices needed for the image acquisition are at the Imaging Center Essen (EMU Dr. Mike Hasenberg).
Publication: Superiority of FIB-SEM tomography of cardiomyocytes over standard two-dimensional analyses highlighted by unmasking mitochondrial heterogeneity. Heinen-Weiler J, Hasenberg M, Heisler M, Settelmeier S, Beerlage A-L, Doepper H, Walkenfort B, Odersky A, Luedike P, Winterhager E, Rassaf T, Hendgen-Cotta UB. J Cachexia, Sarcopenia, Muscle. 2021
https://pubmed.ncbi.nlm.nih.gov/34120411/
Contact:
Prof. Dr. rer. nat.
Elke Winterhager
Light sheet fluorescence microscopy / Histology
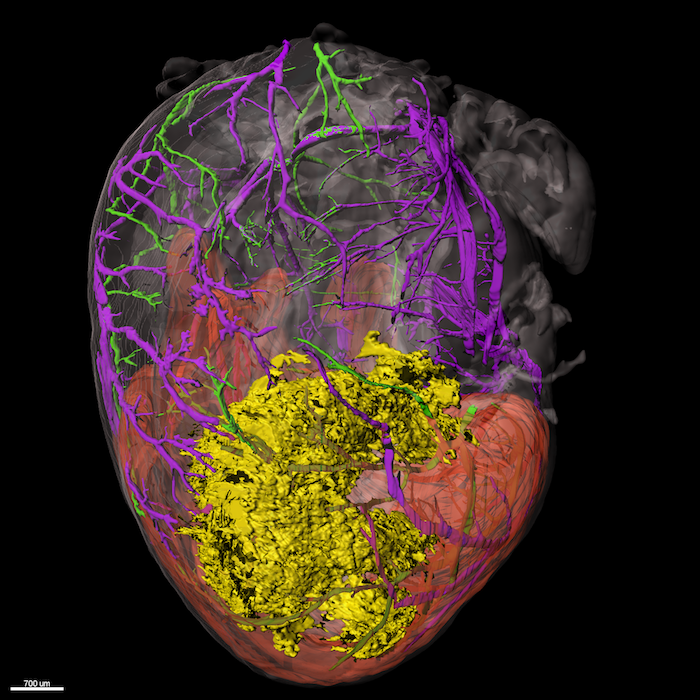
Light sheet fluorescence microscopy: Imaging of biological samples has been long done in 2D, with only recently adding the height of the sample when analyzing subcellular components using techniques like confocal microscopy. Imaging of larger samples, nevertheless whole organs was impossible due to light scattering at the diverse contents of cells making organs opaque.
In recent years, clearing techniques have seen a rise in popularity in order to make such bigger samples imageable. In combination with development of light sheet microscopes, allowing a wide range illumination and z scanning of the sample, it is possible to acquire 3D data sets of whole organs with low time and effort.
In our lab, we employ ethyl cinnamate-based clearing, which on the one hand is not toxic as many other clearing protocols and on the other hand preserves fluorophores allowing for antibody staining. This staining can be done in vivo via tail vein injection or ex vivo by whole mount staining of the extracted organ. In such way, targets of interest can be visualized in 3D inside the heart, which for example allows for a spatial illustration of the damage extent after myocardial infarction, as well as a co-localization of immune cells. For imaging, we use the UltraMicroscope II or UltraMicroscope Blaze provided by the Imaging Center Essen (IMCES).
Histology: Cardiac damage manifests in changes of heart tissue architecture and protein expression. In our lab we employ tried and true histological and immuno stainings to get to the bottom of the damage pathways at hand. We use state-of-the-art imaging methods to unravel subcellular constellation following infarction.
This includes traditional histological stainings like hematoxylin/eosin and gomori trichrome, as well as fluorescence- and chromatogen-based antibody staining. For visualization our lab is equipped with basic light and fluorescence microscopes. For subcellular investigation, we use the devices provided by the Imaging Center Essen (IMCES) like the Leica TCS SP8, the Zeiss Elyra and the Zeiss Apotome.
Contact:
Dr. rer. nat.
Sebastian Korste
Dr. med.
Elias Haj-Yehia
Mouse Phenotyping
There have been major advances in the understanding of cardiac biology in the healthy and failing myocardium, but these are, as yet, unmatched by advances in therapeutics. According to the etiology of a cardiac disorder, surgical models are based on various methods allowing for the narrowing or occlusion of coronary arteries.Depending on the duration and extent of the impairment of coronary blood flow and its consequences for cardiac tissue, these are classified as models of myocardial infarction, cardiac ischemia/reperfusion injury, or chronic cardiac ischemia. Mouse models of heart failure and adaptations in these models may allow easier translation of animal experimentation into the clinical arena.
In addition, factors such as species, strain, and gender of the laboratory animals also significantly contribute to the pathophysiology of the induced disorder and, therefore, have to be taken into consideration thoroughly when an animal model is to be established.
Techniques include:
- in vivo open- and closed-chest surgical models of myocardial infarction (acute and permanent occlusion)
- in vivo surgical model of myocardial ischemia/reperfusion (I/R) injury
- in vivo surgical model of hind limb ischemia
- in vivo hypoxia
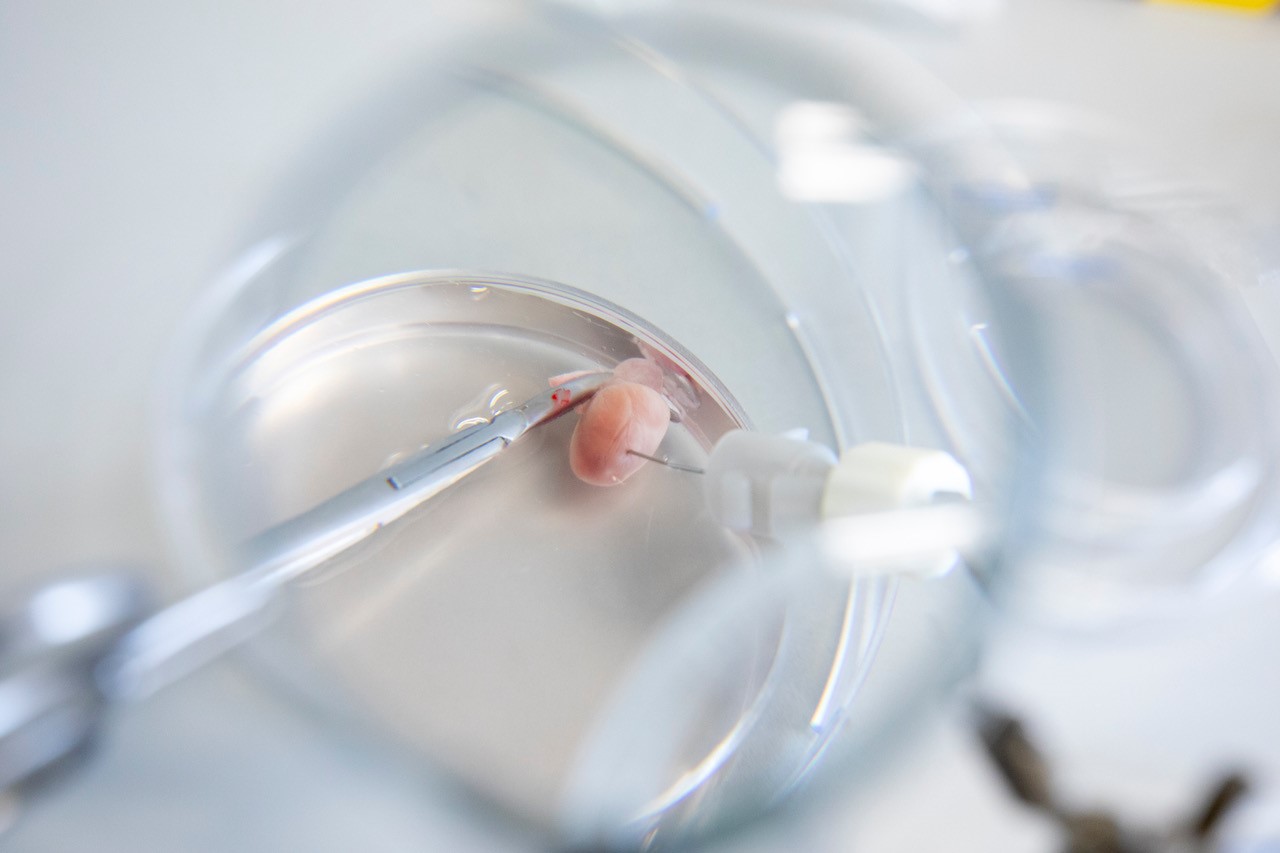
- ex vivo cardiovascular response (isolated heart – Langendorff model)
- blood pressure analysis
- ex vivo organ perfusion analysis
Publication: Revealing subtle changes in cardiac function using transthoracic dobutamine stress echocardiography in mice. Settelmeier S, Rassaf T, Hendgen-Cotta, UB. J Vis Exp. 2021;(168)
https://pubmed.ncbi.nlm.nih.gov/33645572/
Contact:
Dipl.-Biol.
Pia Stock
Cell Culture / Flow Cytometry
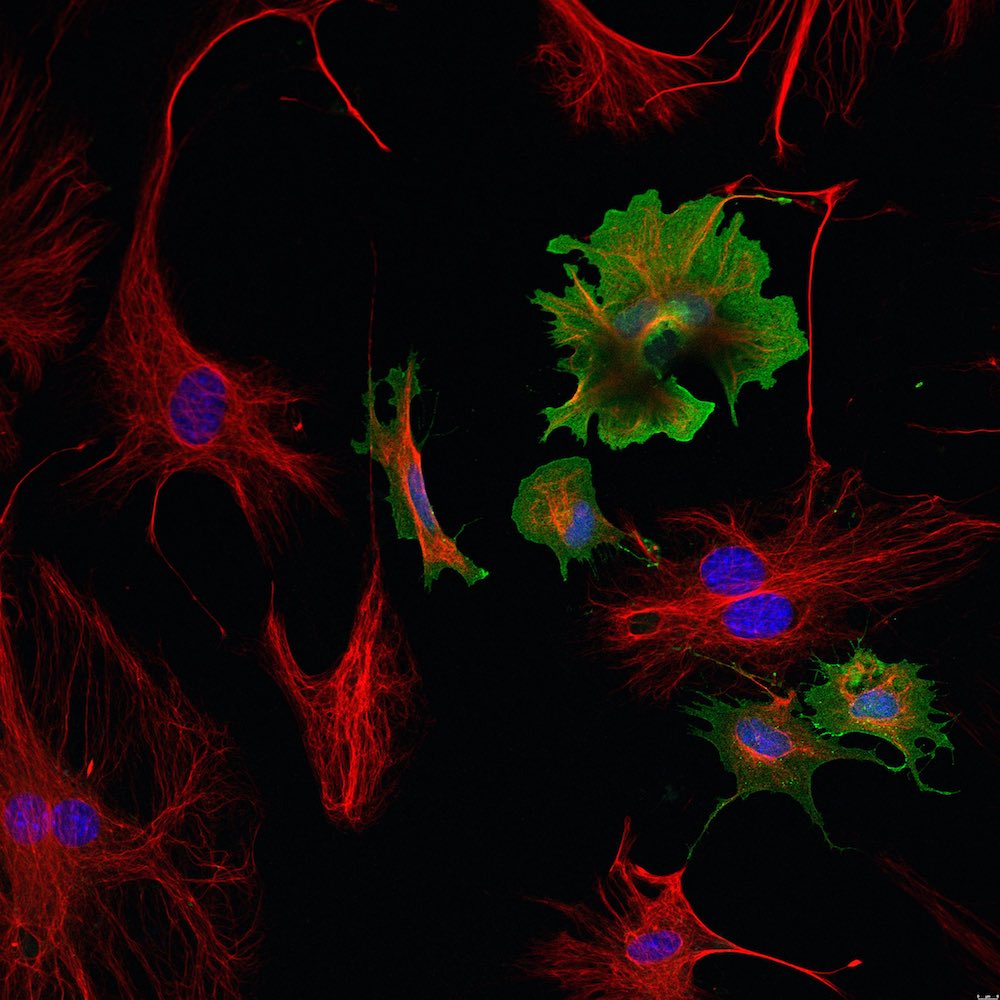
Cell culture
Cell culture is a very versatile tool to study cell types in an artificially controlled environment. There are mainly two different types of cell culture: primary cells and (immortalized) cell lines, which are often modified to survive for extended periods of time. Our group established a reliable method to isolate cardiomyocytes, cardiac fibroblasts and endothelial cells simultaneously from the same adult mouse heart. These cells can be used for a variety of experiments and can be easily isolated from different genetic mouse models.
Furthermore we work with adult cardiomyocytes from human donors and human iPSC-derived cardiomyocytes, which start to beat spontaneously in culture. In comparison to adult mouse cardiomyocytes these cells can be cultured for longer time periods.
Our cells are analyzed with various methods:
- immunofluorescence and confocal microscopy
- proximity ligation assay
- western blot
- isolation of mitochondria and seahorse
- electron microscopy
The devices needed for the image acquisition are at the Imaging Center Essen (IMCES).
Contact:
Dr. rer. nat.
Yanis Mouloud
Dr. med.
Elias Haj-Yehia
Flow cytometry
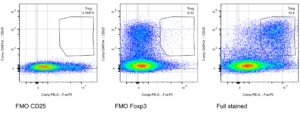
Flow cytometry is a valuable tool to analyze a specific cell population. We conduct multicolor flow cytometry according to standardized protocols. All antibodies that we are using are first titrated. To achieve a high level of reliable data we are always using fluorescence minus one controls in order to determine where the gates should be set.
Regulatory T-cells isolated from murine spleen. Murine regulatory T-cells are characterized by the expression of the surface marker CD25+ and the transcription factor FoxP3+. Splenocytes were isolated and gated on lymphocytes -> single cells -> live cells -> CD3 -> CD4. According to the FMO controls gates were set to identify the double positive cell population.
Proteomics and Protein Dynamics
The functionality of proteins is foremost determined by their conformational state, posttranslational modifications, protein-protein interactions and their microenvironment. Understanding protein function and elucidating alterations under pathological conditions is essential to gain detailed insights into various cardiovascular diseases. We use different, complementary methods to accomplish this task, including:
- Protein expression and purification
- Purification of cellular compartments (cytosolic fractions and mitochondria)
- Denaturing and native gel-electrophoresis
- 2D-gel-electrophoresis
- Co-immunoprecipitation
- Western blotting and immunodetection (fluorescent and chemiluminescent)
- Enzyme-linked immunosorbent assays
- Size-exclusion chromatography
- Flow cytometry and fluorescently activated cell sorting
Contact:
Andrea Odersky
Dr. rer. nat.
Yanis Mouloud
Molecular Genetics
Differential expression of DNA/RNA manifests the cellular phenotype. Thus, introducing genetic modifications to organisms, studying the transcriptome and amending this knowledge by investigating the microenvironment, molecular genetics offer implementations to address the base of cardiovascular diseases. We benefit from various methods:
- RNA and DNA isolation
- RNA and DNA measurements
- siRNA gene silencing
- Genotyping with polymerase chain reaction (PCR)
- Reverse transcription and quantitative real-time RT-PCR (SYBR® Green and TaqMan® based)
- Establishment of transgenic mouse models
- Vector based protein expression systems
Contact:
Christoph Jansen
Energy Metabolism
Real-Time Cell Metabolic Analysis
Mammalian cells generate ATP by mitochondrial (oxidative phosphorylation) and non-mitochondrial (glycolysis) metabolism. To assess the energy metabolism, we evaluate the oxygen consumption rate in isolated mitochondria as well as the oxygen and proton flux over time in living cells, utilizing the Seahorse XFe 24 Extracellular Flux Analyzer. Modulators of key components of the electron transport chain and the glycolytic pathway provide insights into the mechanism of mitochondrial dysfunction.
Contact:
Andrea Odersky
Nitric Oxide Analytics
Nitric oxide (NO) has been established as an important regulator of cardiovascular functions. Besides modulation of the basal vascular tone, NO governs myocardial contractility, prevents platelet activation, and limits leukocyte adhesion to the endothelium. Endogenous and exogenous nitrate and nitrite represent a bioavailable storage pool of NO that is bioactivated along the physiological oxygen gradient to regulate a number of vascular and cellular responses like hypoxic vasodilation, cardiac energetics, mitochondrial electron transfer, and calcium handling.
To assess the NO pool in tissue and fluids, we detect NO chemically and specifically in the gas phase by the chemiluminescence of its reaction with ozone and nitrate and nitrite with a specialized HPCL system.
Contact:
Andrea Odersky
Dr. med.
Fabrice Reyes